- A cat in a box.
This subject is very complex and at the same time something simple. If you are a person oblivious to science like physics or chemical this can be weird and strange. Don’t worry, I just suggest you to avoid all mathematical equation of this blog.
Who hasn’t heard of the Schrödinger’s cat? I always listen to people talk about a cat in a box. Maybe this isn’t new for you, but you really can say me what is the meaning for that?
Ok, let’s start. First, Schrödinger was an Austrian physicist. He made a famous equation in 1925, possibly the most important one for quantum physics.
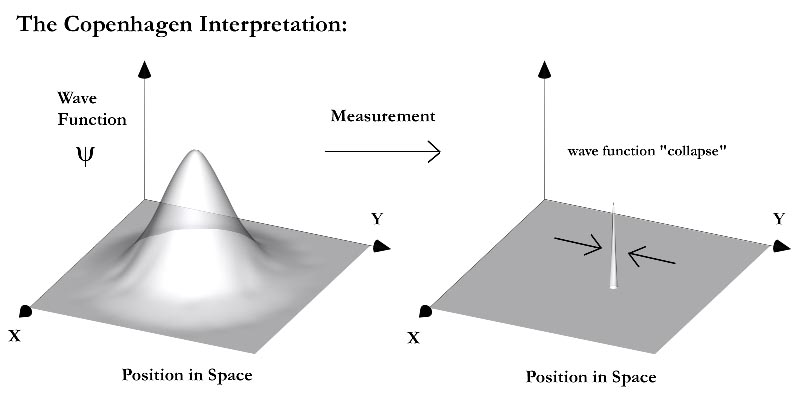
Shrödinger invented an analogy with his equation and a cat in a box, because his equation tries to find the probability of seeing an electron in a place in space. At the same way that in his analogy we can find a cat alive or dead inside a box. So, what is the interesting? In fact, probability is the key. Shrödinger said that as long as you didn’t open the box, you wouldn’t know if the cat was alive or dead and in moment you open the box, you can know. In general, this equation tells us that we will never know the location of the particle just a probability of where it might be.

Now, this part is something hard. Do you know that electrons have a property of being able to stay in 2 places at the same time? Yes, I know. Now you understand better, do not? The cat be able to stay alive or dead. This phenomenon is called superposition.
The next episode I will talk about Schrödinger’s equation more specifically its application for describing stationary waves (atomic orbitals) for a combination of the numbers quantum of hydrogen. [Only recommended for people with mathematical knowledge]