This topic is related to different processes in our daily lives (like all topics in this blog). For example, in a restaurant when we order hot coffee whenever time passes it start to cool and it’s normal, no?When I think about that the zeroth law of thermodynamics comes to my mind. It is very intuitive, it is not necessary to be a genius to understand it.
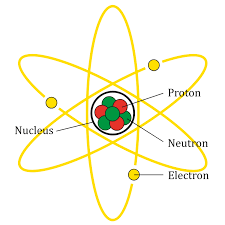
Before, we had talked about thermal equilibrium and the zeroth law of thermodynamics talks about this (Figure 1). The equilibrium says: if we have two objects whit different temperatures and are in contact, it will exist as long as time passes a thermal equilibrium where both objects will be at the same temperature.
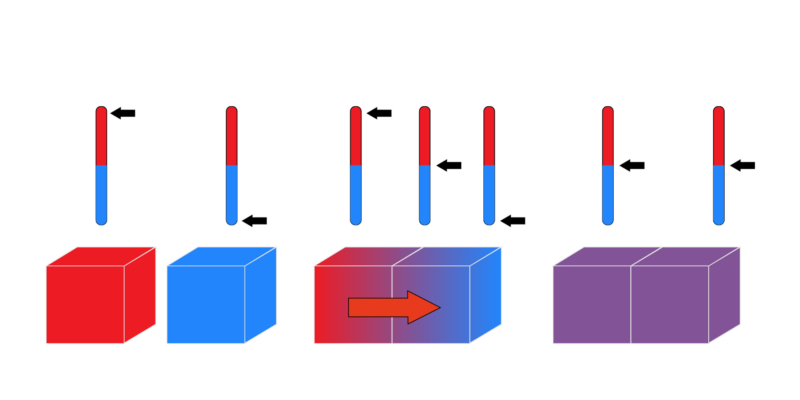
Therefore, the law says: if two systems A and B are separated from each other in thermal equilibrium with a third system called C (next to B but not next to A), then A and C are in thermal equilibrium with each other (Figure 1.5).

In the case of the coffee cup (Figure 2), the air is the other element in heat transfer. It’s impossible for the atmosphere to increase its temperature, normally the coffee decreases its temperature equal to the atmosphere (equilibrium). Why? First I want to announce two things:
1.- Everyone in this world works with physics and chymics, I mean the temperature is associated with the kinetic energy of particles (atoms, molecules…) in the system, in termodinamics it is called internal energy.
2.- There are different forms of heat transfer such as diffusion, convection and radiation.
It’s important to know that they exist, even if you don’t understand it don’t worry, later I will tell you.

Now, let’s talk about different equations that describe heat transfer. When we heat a substance, we can observe that the temperature increases, this is because the first system (for example a flame) transports heat continously to second system (like air in atmosphere with less temperature), this heat flow (Q, Cal) represents the amount of energy in the form of heat that is transferred over an area (q, Cal/m^2). Intuitively, It could be assumed that a constant heat flow (first system) increases the temperature of another second system over time, but this will be conditioned by the temperature by the both system. But if the heat flow (first system) is not constant like a cup of coffee in a restaurant, then the second system (air in atmosphere) will cool the first system. Experimently, Newton found the following:

First, this only works between solids and fluids, when Newton discovered this phenomenon he only experimented with these states of matter. Second, Newton only thought of a small hot system with Ts in contact with a large cold system with Tf like a cup of coffee in the outdoor environment (and yes, the amount of mass is important). Third, Newton understood that this exchange was different for each substance, so he introduced a constant called heat exchange coefficient “h” (Figure 4).
Newton to remove that proportion with “h”:
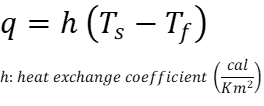
“h” depends on each fluid and contact area, it represents heat transfer by convection. Rearranging:
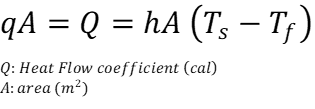
At the same time, analogously to the previous example we can deduce its dependence on time.

The question now is how. Always when one system loses heat another system gains it, this is a heat balance, and in theory they will be equal. In termodinamics this is relationed whit the entalpy, but for this moment just you belive me whit the following:
1) If the temperature Ts of the system is higher than the ambient temperature Tf, the body loses an amount of heat dQ. From the energy balance we know that the heat flow depends proportionally on mass, temperature and specific heat capacity. Finally, specific heat capacity is the ability of a body to store heat. Don’t worry, in the second chapter we will go deeper into this topic about energy balance and enthalpy.
2) This expression refers specifically to Newton’s law of cooling.
3) With this equation, you can show an approximation of how long it takes for your coffee cup to cool.
* To remember: this equation is for convection transport and have important assumptions to use.
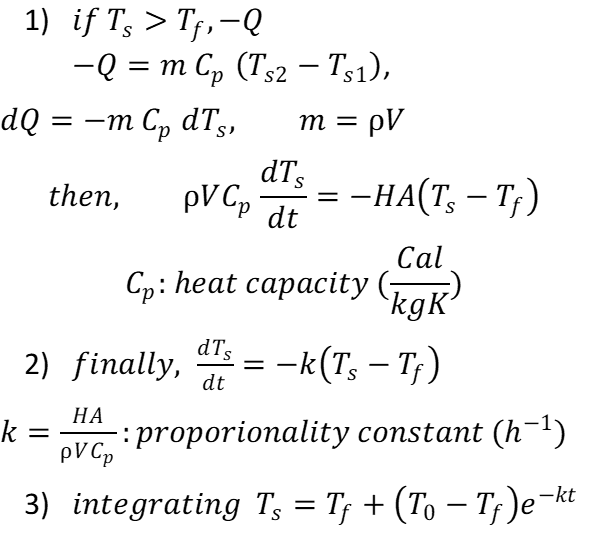
I know that this last part was very fast, but the important thing is to understand that this type of phenomenon is not difficult to model. In the next chapter on this topic, I will explain how to use the last equation and show you other equations and their application in the industry, more specifically in heat exchange (process equipment in the chemical industry).
See you soon!!! Thank you 🙂